ABSTRACT:
In this article, we will discuss about the molecular and genetic basis of cystic fibrosis. It effects the respiratory system and digestive system system. Cystic fibrosis cause due to mutation in gene that regulate the transmembrane conduction during respiration and digestion. We will discuss the molecular pathways and genetic basis of cystic fibrosis. We will also discuss the potential treatment of the disease.
INTRODUCTION:
Cystic fibrosis (CF) is a life-threatening genetic disorder that primarily affects the lungs and digestive system. It is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, leading to the production of a faulty CFTR protein. Understanding the molecular and genetic basis of CF is crucial for developing effective treatments and improving the quality of life for individuals living with this condition. This article aims to explore the current knowledge on the molecular and genetic aspects of CF, highlighting key research findings and their implications.
GENETIC BASIS OF CYSTIC FIBROSIS:
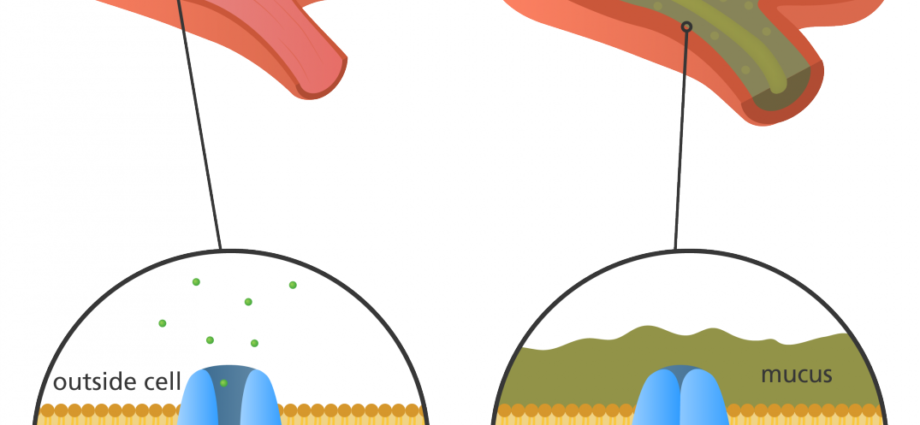
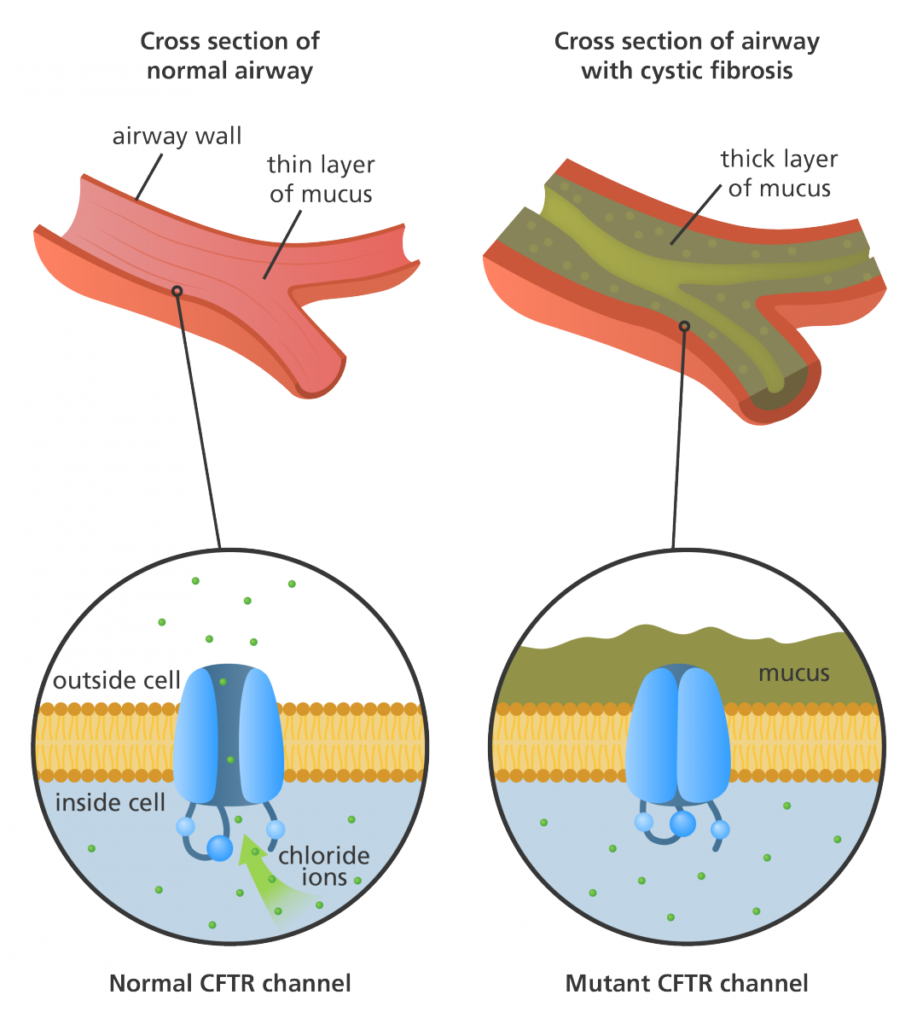
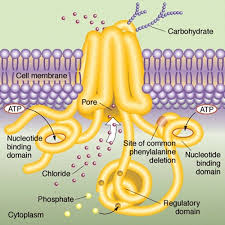
The CFTR gene, located on chromosome 7, encodes the CFTR protein, which functions as a chloride channel on the surface of epithelial cells. Mutations in the CFTR gene can disrupt the normal functioning of this protein, leading to the development of CF. Over 2,000 different mutations identified in the CFTR gene, with the most common mutation being the deletion of phenylalanine at position 508 (F508del). This mutation accounts for approximately 70% of CF cases worldwide.
MOLECULAR BASIS OF CYSTIC FIBROSIS:
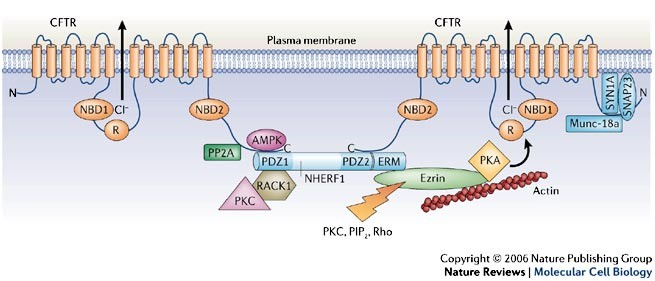
The CFTR protein plays a crucial role in maintaining the balance of salt and water in various organs. It also effects the lungs and pancreas. In healthy individuals, CFTR allows chloride ions to pass through the cell membrane, regulating the movement of water. However, in CF patients, the defective CFTR protein disrupts this process, resulting in production of thick, sticky mucus in airways. So the result is impaired pancreatic function.
The F508del mutation, in particular, leads to the misfolding and degradation of the CFTR protein within the cell. This results in reduced levels of functional CFTR on the cell surface, further impairing chloride ion transport. Additionally, the abnormal CFTR protein can also disrupt the function of other ion channels and transporters, contributing to the overall pathology of CF.
RESEARCH ADVANCES:
Significant progress has been made in understanding the molecular and genetic basis of CF. It leading to the development of targeted therapies. One such breakthrough is the approval of CFTR modulator drugs, such as ivacaftor, lumacaftor, and tezacaftor. They aim to correct the underlying CFTR protein defects. These drugs have shown promising results in improving lung function and reducing disease progression in CF patients with specific mutations.
Furthermore, advancements in gene editing technologies, such as CRISPR-Cas9, hold great potential for correcting CF-causing mutations directly in the patient’s DNA. Although still in the early stages of development, these approaches offer hope for a future where CF can be treated at its genetic root.
CONCLUSION:
Understanding the molecular and genetic basis of cystic fibrosis has paved the way for targeted therapies and potential gene-editing strategies. The identification of CFTR gene mutations and the subsequent development of CFTR modulator drugs have revolutionized CF treatment, significantly improving the quality of life for affected individuals. Continued research in this field will undoubtedly lead to further breakthroughs, ultimately aiming to find a cure for this debilitating genetic disorder.
REFERENCES:
Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16(1):45-56. https://www.nature.com/articles/nrg3849
Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992-2001. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7041544
Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663-1672. https://www.nejm.org/doi/full/10.1056/nejmoa1105185
Clancy JP, Rowe SM, Accurso FJ, et al. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax. 2012;67(1):12-18. https://experts.umn.edu/en/publications/results-of-a-phase-iia-study-of-vx-809-an-investigational-cftr-co