ABSTRACT:
In this article, we will discuss about the synthesis and reactions of hydrocarbons. Hydrocarbons are mainly of two types: saturated and unsaturated hydrocarbons. These are further divided into several types such as, acyclic, alicyclic, aromatic, etc. We will also discuss the mechanisms of these reactions to understand the concepts. We will provide references to support the topic details.
INTRODUCTION:
Alkanes, also known as saturated hydrocarbons, are organic compounds consisting solely of carbon and hydrogen atoms. They are the simplest and most abundant class of hydrocarbons, with a wide range of applications in various industries. Understanding the preparations and reactions of alkanes, including their mechanisms, is crucial for their synthesis and utilization in different fields.
SYNTHESIS AND REACTIONS OF ALKANES:
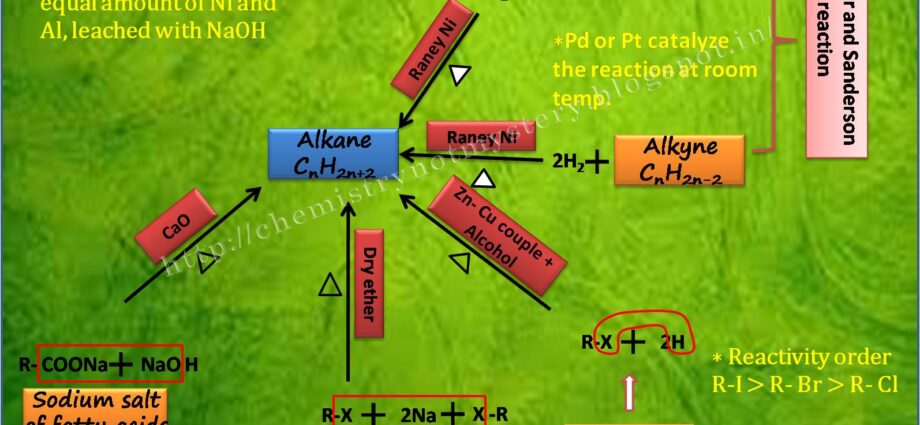
1. PREPARATIONS OF ALKANES:
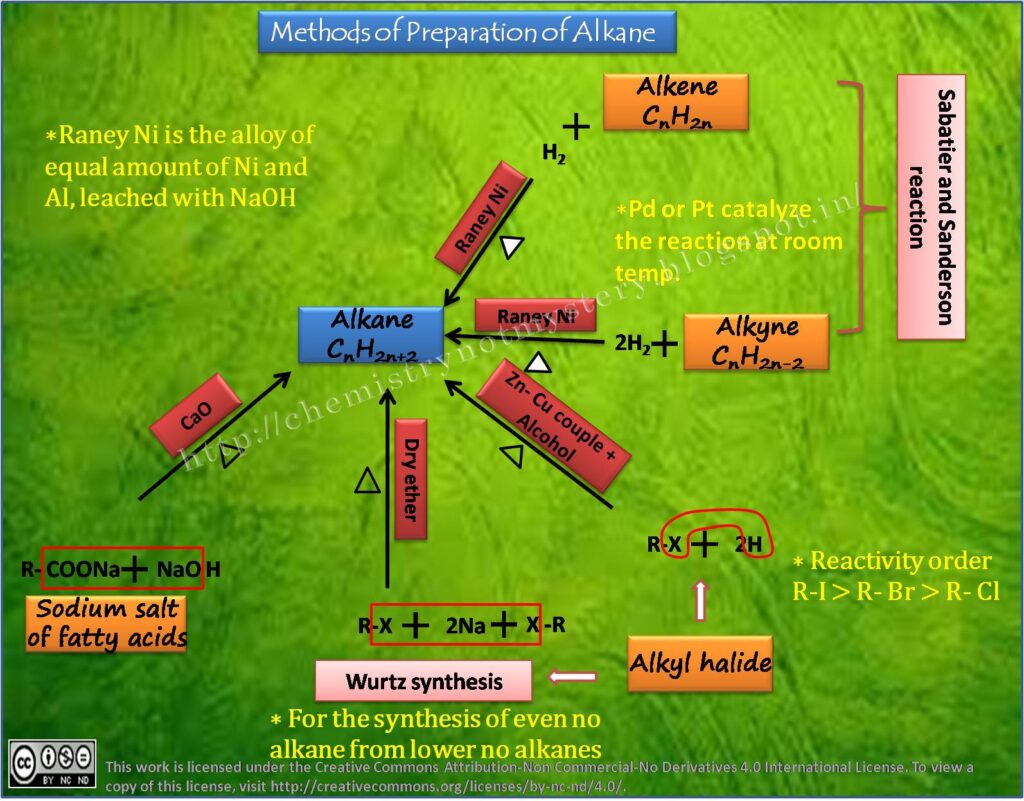
a) REDUCTION OF ALKENES OR ALKYNES:
Alkanes can be prepared by the reduction of alkenes or alkynes. This reaction involves the addition of hydrogen (H2) in the presence of a catalyst, such as palladium or platinum, to break the carbon-carbon double or triple bond.
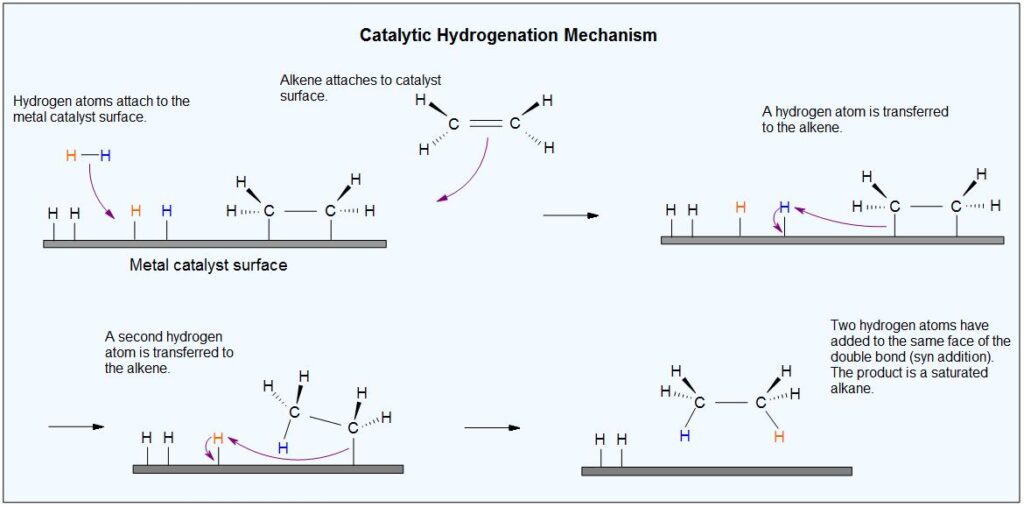
b) DECARBOXYLATION OF CARBOXYLIC ACID:
Another method for preparing alkanes is the decarboxylation of carboxylic acids. This reaction involves the loss of carbon dioxide (CO2) from a carboxylic acid, resulting in the formation of an alkane.
c) REDUCTION OF ALKYL HALIDES:
Alkanes can also synthesize by the reduction of alkyl halides, a process known as the Wurtz reaction. In this reaction, an alkyl halide treated with metallic sodium (Na) or potassium (K) in an anhydrous solvent, resulting in the formation of an alkane. The mechanism involves the formation of a free radical intermediate, followed by the recombination of two alkyl radicals to form the desired alkane.
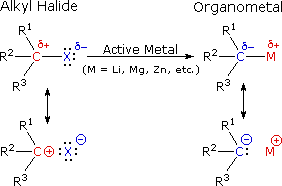
2. REACTIONS OF ALKANES:
a) COMBUSTION:
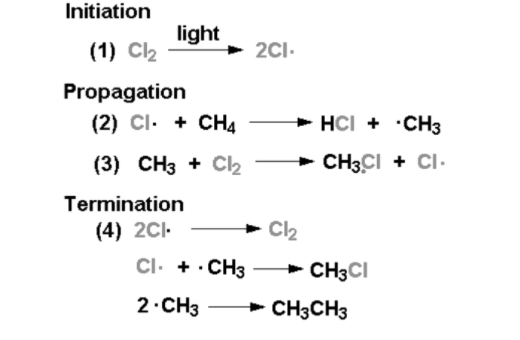
Alkanes readily undergo combustion in the presence of oxygen, producing carbon dioxide (CO2) and water (H2O) as the main products. This exothermic reaction is highly utilized in energy production, as alkanes serve as fuels in various combustion engines. The combustion of alkanes follows a radical mechanism, involving the initiation, propagation, and termination steps.
b) HALOGENATION:
Alkanes can undergo halogenation reactions with halogens, such as chlorine (Cl2) or bromine (Br2), in the presence of light or heat. This reaction involves the substitution of a hydrogen atom in the alkane with a halogen atom. The halogenation of alkanes proceeds via a radical mechanism, where the halogen radicals abstract a hydrogen atom, leading to the formation of a halogenated alkane and a new halogen radical.
c) DEHYDROGENATION:
Alkanes can undergo dehydrogenation reactions to form alkenes. This reaction involves the removal of hydrogen atoms from adjacent carbon atoms, resulting in the formation of a carbon-carbon double bond. Dehydrogenation reactions are typically carried out using high temperatures and catalysts, such as platinum or palladium. The mechanism of dehydrogenation involves the formation of a carbon-hydrogen bond, followed by the elimination of hydrogen gas.

CONCLUSION OF SYNTHESIS AND REACTIONS OF ALKANES:
Preparations and reactions of alkanes play a vital role in the synthesis and utilization of these compounds in various industries. Understanding the mechanisms involved in these processes allows researchers and scientists to develop efficient strategies for the production and modification of alkanes. By employing different methods, such as hydrogenation, reduction, and decarboxylation, alkanes can be prepared from various starting materials. Additionally, reactions like combustion, halogenation, and dehydrogenation provide avenues for the transformation of alkanes into valuable products. Further research and exploration of these mechanisms will continue to enhance our understanding and utilization of alkanes.
REFERENCES:
Clayden, J., Greeves, N., Warren, S., & Wothers, P. (2012). Organic Chemistry. Oxford University Press. https://www.chemcome.com/wp-content/uploads/2020/11/Organic-Chemistry-by-Jonathan-Clayden-Nick-Greeves-Stuart-Warren-z-lib.org_.pdf
Smith, M. B., & March, J. (2007). March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. John Wiley & Sons.
Klein, D. R. (2011). Organic Chemistry as a Second Language: First Semester Topics. John Wiley & Sons. https://www.wiley.com/en-ie/Organic+Chemistry+as+a+Second+Language:+First+Semester+Topics,+5th+Edition-p-9781119493488
McMurry, J. (2015). Organic Chemistry (9th ed.). Cengage Learning.
Carey, F. A., & Giuliano, R. M. (2017). Organic Chemistry (10th ed.). McGraw-Hill Education.
March, J. (2013). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.). Wiley-Interscience.