ABSTRACT:
In this article, we will discuss about the geometries of transition metal complexes. Transition metal complexes are fascinating structures that exhibit a wide range of geometries due to the unique electronic configurations of transition metals. Understanding the geometries of these complexes is crucial in predicting their reactivity and properties. In order to comprehend the geometries of transition metal complexes, it is essential to first grasp the concept of coordination number and the types of ligands that can bind to transition metals. The coordination number refers to the number of ligands surrounding the central transition metal ion. This number can vary from two to twelve, resulting in different geometries such as linear, square planar, tetrahedral, trigonal bipyramidal, and octahedral. Additionally, the types of ligands that bind to transition metals greatly influence their geometry.
INTRODUCTION:
Transition metal complexes play a crucial role in various fields, including catalysis, materials science, and bioinorganic chemistry. The geometries of these complexes are of utmost importance as they determine their reactivity, stability, and electronic properties. In this article, we will explore the different geometries exhibited by transition metal complexes, highlighting their structural features and discussing their implications in various applications.
1. OCTAHEDRAL GEOMETRY:
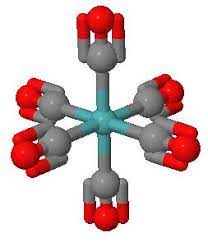
Octahedral geometry one of the most common geometries observed in transition metal complexes. In this arrangement, the central metal ion surrounded by six ligands, forming an octahedron. The ligands can be either monodentate or polydentate, and they occupy the six coordination sites around the metal ion. Octahedral complexes often found with transition metals such as iron, cobalt, and nickel. They exhibit high symmetry and known for their stability and inertness.

2. SQUARE PLANAR GEOMETRY:
Square planar geometry another important geometry observed in transition metal complexes. In this arrangement, the central metal ion coordinated by four ligands, forming a flat square plane. Square planar complexes commonly found with transition metals such as platinum, palladium, and gold. These complexes often exhibit unique electronic properties due to the presence of d-orbitals that involved in bonding.

3. TETRAHEDRAL GEOMETRY:
Tetrahedral geometry is less common than octahedral and square planar geometries but still observed in certain transition metal complexes. In this arrangement, the central metal ion coordinated by four ligands, forming a tetrahedron. Tetrahedral complexes often found with transition metals such as manganese, chromium, and vanadium. These complexes can exhibit different reactivity and electronic properties compared to their octahedral or square planar counterparts.

4. TRIGONAL BIPYRAMIDAL GEOMETRY:
Trigonal bipyramidal geometry observed when a transition metal ion coordinated by five ligands. In this arrangement, three ligands occupy the equatorial plane, while the remaining two ligands occupy the axial positions. Trigonal bipyramidal complexes commonly found with transition metals such as molybdenum, tungsten, and rhenium. These complexes often exhibit unique reactivity due to the presence of different ligand environments.

5. SQUARE PYRAMIDAL GEOMETRY:
Square pyramidal geometry is a variation of the trigonal bipyramidal geometry. In this arrangement, the central metal ion coordinated by five ligands, with four ligands occupying the equatorial plane and one ligand occupying the axial position. Square pyramidal complexes are less common but have observed with transition metals such as copper, silver, and gold. These complexes often exhibit interesting electronic properties due to the presence of different ligand environments.

CONCLUSION:
The geometries of transition metal complexes play a crucial role in determining their reactivity, stability, and electronic properties. Octahedral, square planar, tetrahedral, trigonal bipyramidal, and square pyramidal geometries are commonly observed in transition metal complexes, each with its own unique structural features and implications. Understanding these geometries is essential for designing and studying transition metal complexes in various applications, including catalysis, materials science, and bioinorganic chemistry.
REFERENCES:
Hartwig JF. Organotransition Metal Chemistry: From Bonding to Catalysis, 1st ed. Sausalito: University Science Books; 2010.
van Leeuwen PWNM, Kamer PCJ, Reek JNH, Dierkes P. Chem. Rev. 2000;100:2741 https://pubmed.ncbi.nlm.nih.gov/11749304/
Birkholz née Gensow M-N, Freixa Z, van Leeuwen PWNM. Chem. Soc. Rev. 2009;38:1099. https://pubs.rsc.org/en/content/articlelanding/2009/cs/b806211k
van Zeist W-J, Bickelhaupt FM. Dalton Trans. 2011;40:3028. https://pubs.rsc.org/en/content/articlelanding/2011/dt/c0dt01550d

