ABSTRACT:
In this article, we will discuss about the fascinating mechanism of ETC (Electron transport chain reaction). The electron transport chain (ETC) is a vital process that occurs within the mitochondria of eukaryotic cells. It plays a crucial role in cellular respiration, the process by which cells convert nutrients into usable energy. We will discuss about the different regulations of the reaction. We will also provide references to understand the concept deeply.
INTRODUCTION OF MECHANISM OF ETC:
The electron transport chain (ETC) is a crucial process that occurs within the mitochondria of eukaryotic cells. It plays a vital role in the production of adenosine triphosphate (ATP), the energy currency of the cell. The ETC is a complex series of reactions that involve the transfer of electrons through a series of protein complexes. It ultimately leading to the production of adenosine triphosphate (ATP), the energy currency of the cell. Understanding the intricate mechanism and regulation of the ETC is crucial for comprehending the fundamental processes of cellular respiration and energy production.
MECHANISM OF ETC (ELECTRON TRANSPORT CHAIN REACTION):
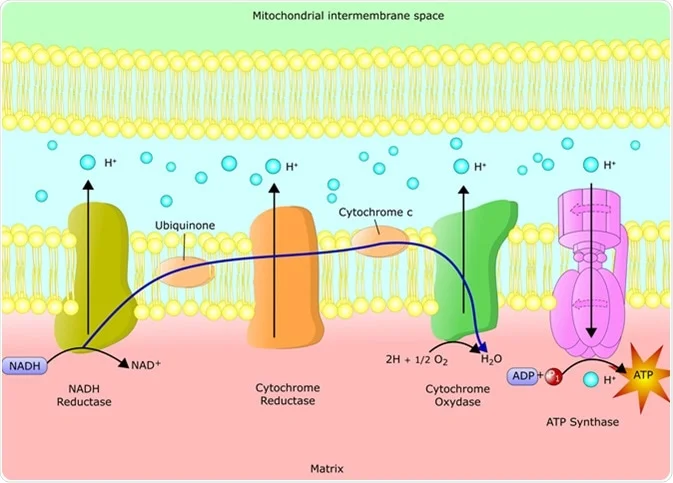
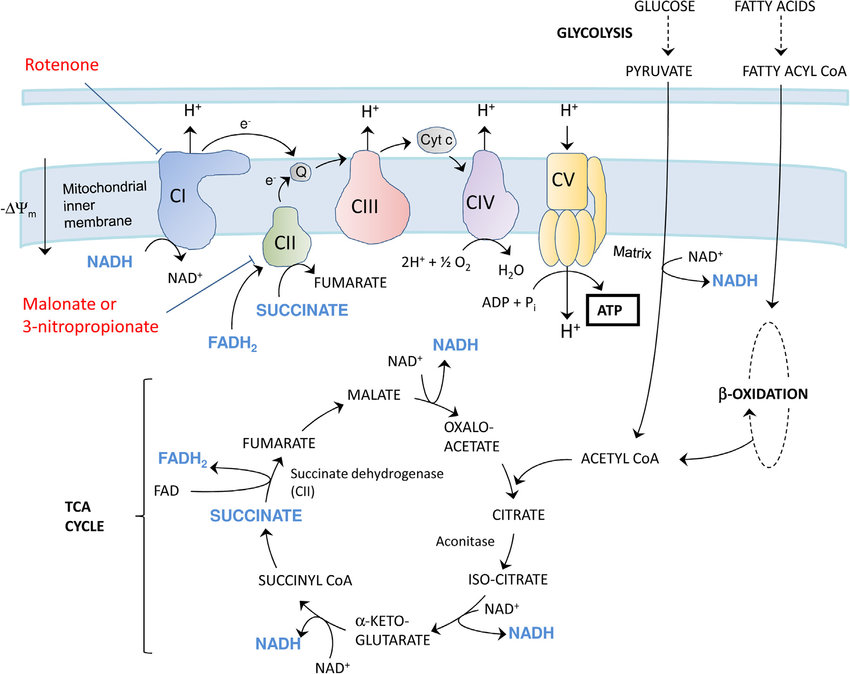
The ETC consists of a series of protein complexes embedded within the inner mitochondrial membrane. These complexes, labeled I to IV, work in tandem to facilitate the transfer of electrons from electron donors to electron acceptors, ultimately leading to the production of ATP.
1. COMPLEX I-NADH DEHYDROGENASE:
NADH, a product of the citric acid cycle, donates electrons to complex I. This complex transfers the electrons to coenzyme Q (CoQ), while simultaneously pumping protons across the inner mitochondrial membrane.
2. COMPLEX II-SUCCINATE DEHYDROGENASE AND COENZYME Q:
CoQ, also known as ubiquinone, acts as a mobile electron carrier. It accepts electrons from complex I and transfers them to complex III.
3. COMPLEX III-CYTOCHROME BC1 COMPLEX:
Complex III receives electrons from CoQ and transfers them to cytochrome c, another mobile electron carrier. This complex also pumps protons across the inner mitochondrial membrane.
4. CYTOCHROME C:
Cytochrome c shuttles electrons from complex III to complex IV.
5. COMPLEX IV-CYTOCHROME C OXIDASE:
Complex IV receives electrons from cytochrome c and transfers them to molecular oxygen (O2), resulting in the formation of water (H2O). This complex also contributes to proton pumping.
6. COMPLEX V-ATP SYNTHASE:
The electrochemical gradient generated by the ETC is harnessed by ATP synthase, a protein complex located in the inner mitochondrial membrane. ATP synthase utilizes the energy from the proton gradient to convert adenosine diphosphate (ADP) and inorganic phosphate (Pi) into ATP through a process called oxidative phosphorylation. This final step in the ETC results in the production of ATP, which is then utilized by the cell for various energy-requiring processes.
REGULATION OF MECHANISM OF ETC:
The ETC is tightly regulated to maintain optimal energy production and prevent the accumulation of harmful reactive oxygen species (ROS). Several regulatory mechanisms ensure the efficient functioning of the ETC:
1. SUBSTRATE AVAILABILITY:
The availability of electron donors, such as NADH and FADH2, directly influences the rate of electron flow through the ETC. The regulation of substrate availability is primarily controlled by the citric acid cycle and glycolysis.

2. FEEDBACK INHIBITION OF MECHANISM OF ETC:
The ETC is subject to feedback inhibition by ATP and NADH. High levels of ATP and NADH inhibit complex I, reducing the flow of electrons through the ETC. This mechanism prevents excessive ATP production and maintains cellular homeostasis.
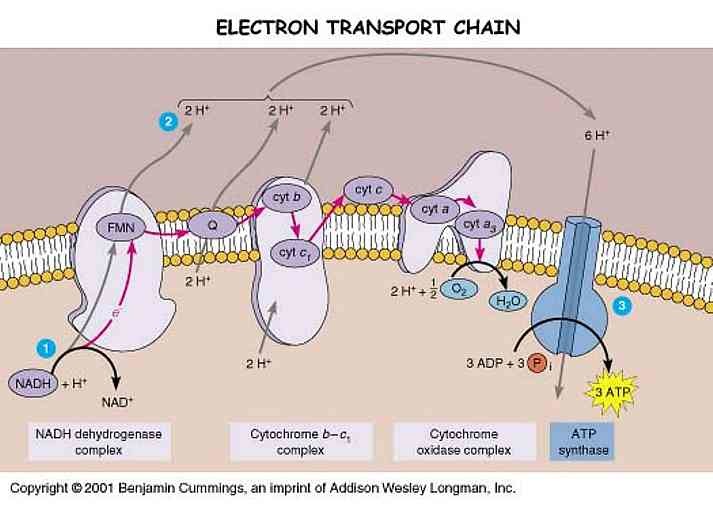
3. PROTON GRADIENT REGULATION OF MECHANISM OF ETC:
The proton gradient across the inner mitochondrial membrane is crucial for ATP synthesis. The enzyme ATP synthase, located in complex V, utilizes the proton gradient to generate ATP. The regulation of proton pumping by complexes I, III, and IV ensures the maintenance of an optimal proton gradient.
4. ANTI-OXIDANT DEFENSE MECHANISM OF ETC:
The ETC generates ROS as a byproduct, which can cause cellular damage if not regulated. Antioxidant enzymes, such as superoxide dismutase and catalase, help neutralize ROS and maintain redox balance within the mitochondria.
SIGNIFICANCE OF ETC REACTION:
The primary function of the ETC is to generate ATP through oxidative phosphorylation. As the electrons flow through the protein complexes, the energy released is used to pump protons across the inner mitochondrial membrane. This establishes a proton gradient, with a higher concentration of protons in the intermembrane space compared to the mitochondrial matrix. It is estimated that approximately 90% of ATP is generated through oxidative phosphorylation in the mitochondria. Without a functional ETC, cells would be unable to efficiently produce ATP, leading to a severe energy deficit and impaired cellular function.
CONCLUSION:
The electron transport chain reaction is a complex process that plays a pivotal role in cellular energy production. By transferring electrons and pumping protons, the ETC establishes an electrochemical gradient that drives ATP synthesis. Understanding the intricacies of this process is crucial for comprehending cellular respiration and its implications in various physiological processes. Further research in this field will continue to unravel the mysteries of the electron transport chain and its significance in cellular metabolism.
REFERENCES:
Mitchell, P. (1961). Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature, 191(4784), 144-148. https://www.nature.com/articles/191144a0
Sazanov, L. A. (2015). A giant molecular proton pump: structure and mechanism of respiratory complex I. Nature Reviews Molecular Cell Biology, 16(6), 375-388. https://pubmed.ncbi.nlm.nih.gov/25991374/
Nelson, D. L., Cox, M. M. Lehninger Principles of Biochemistry. 7th edition. W.H. Freeman and Company, 2017. https://www.scirp.org/(S(czeh2tfqyw2orz553k1w0r45))/reference/ReferencesPapers.aspx?ReferenceID=2103234
Nicholls, D. G., & Ferguson, S. J. (2013). Bioenergetics 4. Academic Press. https://www.neuroscience.ox.ac.uk/publications/596143


greetings ! great post .
stay awesome and keep blogging
http://www.pomeranianpuppies.uk