ABSTRACT:
In this article, we will discuss about the fascinating mechanism of gluconeogenesis. Gluconeogenesis is the metabolic pathway to synthesize glucose from non-carbohydrate precursors, such as amino acids, glycerol, etc. This process occur in liver and kidneys. It is the back up plan for body to produce energy. We will describe the enzymatic, hormonal and transcriptional regulations of this mechanism. We will also provide references to understand the concept deeply.
INTRODUCTION:
Gluconeogenesis is a vital metabolic pathway that allows the body to produce glucose from non-carbohydrate sources, such as amino acids, lactate, and glycerol. This process plays a crucial role in maintaining blood glucose levels during periods of fasting, intense exercise, or low carbohydrate intake. Gluconeogenesis ensures a steady supply of glucose to fuel the brain, red blood cells, and other glucose-dependent tissues. In this article, we will explore the process of gluconeogenesis, its regulation, and its significance in maintaining energy homeostasis.
MECHANISM OF GLUCONEOGENESIS:
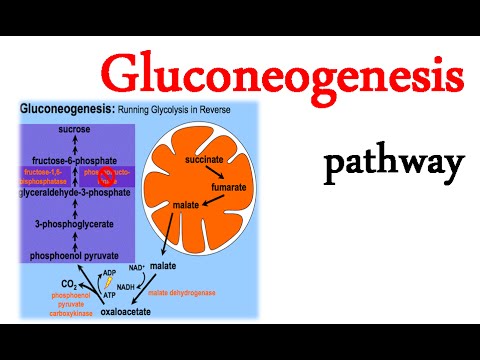
Gluconeogenesis primarily occurs in the liver, although the kidneys also contribute to a lesser extent. The pathway involves a series of enzymatic reactions that convert non-carbohydrate precursors into glucose. The three main substrates for gluconeogenesis are lactate, amino acids (mainly alanine), and glycerol.
1. CONVERSION OF LACTATE TO PYRUVATE:
The first step of gluconeogenesis involves the conversion of lactate to pyruvate, which catalyzed by the enzyme lactate dehydrogenase.
2. CONVERSION OF PYRUVATE TO PHOSPHOENOL PYRUVATE:
Pyruvate, derived from various sources such as lactate or amino acids, carboxylated by pyruvate carboxylase to form oxaloacetate. Oxaloacetate then decarboxylated and phosphorylated by phosphoenolpyruvate carboxykinase (PEPCK) to generate PEP. Amino acids can also serve as precursors for gluconeogenesis. Alanine, in particular, is a major contributor as it released from muscle tissue during periods of prolonged exercise or fasting. Alanine converted to pyruvate through a process called transamination, and then enters the gluconeogenic pathway.
Glycerol, derived from the breakdown of triglycerides in adipose tissue, can also use as a substrate for gluconeogenesis. Glycerol converted to dihydroxyacetone phosphate (DHAP), which then converted to glyceraldehyde-3-phosphate (G3P) and enters the gluconeogenic pathway.
3. CONVERSION OF FRUCTOSE-1,6-BISPHOSPHATE TO FRUCTOSE-6-PHOSPHATE:
Fructose-1,6-bisphosphatase (FBPase) catalyzes the hydrolysis of fructose-1,6-bisphosphate into fructose-6-phosphate.
4. CONVERSION OF GLUCOSE-6-PHOSPHATE TO GLUCOSE:
Glucose-6-phosphatase (G6Pase) catalyzes the dephosphorylation of glucose-6-phosphate to produce glucose, which can be released into the bloodstream.
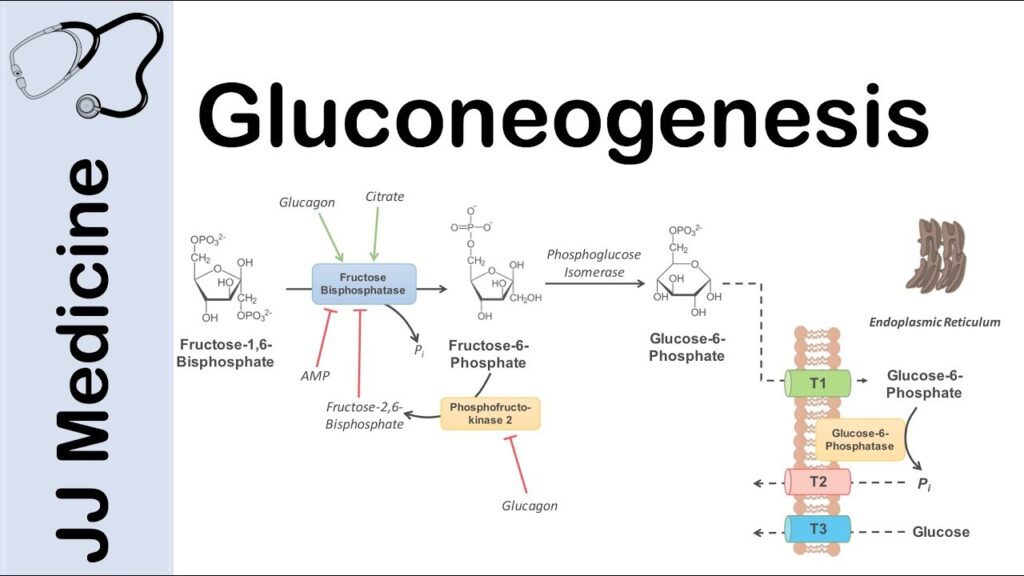
REGULATION OF MECHANISM OF GLUCONEOGENESIS:
Gluconeogenesis is tightly regulated to ensure glucose production matches the body’s energy demands. Several key regulatory factors control the activity of enzymes involved in gluconeogenesis. The major regulators include:
1. HORMONAL REGULATION OF MECHANISM OF GLUCONEOGENESIS:
a) GLUCAGON:
Released by the pancreas in response to low blood glucose levels, glucagon stimulates gluconeogenesis by activating cAMP-dependent protein kinase A (PKA). PKA phosphorylates and activates key enzymes, such as PEPCK and FBPase, promoting gluconeogenesis.
b) INSULIN:
Released by the pancreas in response to high blood glucose levels, insulin inhibits gluconeogenesis by suppressing the expression and activity of key gluconeogenic enzymes.
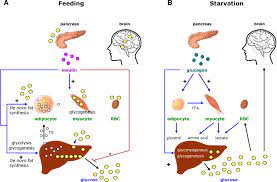
2. SUBSTRATE AVAILABILITY:
a) AMINO ACIDS:
During fasting or starvation, amino acids derived from protein breakdown serve as major precursors for gluconeogenesis.
b) LACTATE:
Produced by anaerobic glycolysis in tissues such as muscles, lactate is transported to the liver, where it is converted to pyruvate and subsequently used for gluconeogenesis.
3. TRANSCRIPTIONAL REGULATION:
Transcription factors, such as CREB, FOXO1, and PGC-1α, play crucial roles in regulating the expression of gluconeogenic enzymes. They respond to hormonal signals and coordinate the expression of genes involved in gluconeogenesis.

SIGNIFICANCE OF MECHANISM OF GLUCONEOGENESIS:
Gluconeogenesis is essential for maintaining blood glucose levels within a narrow range, especially during periods of fasting or low carbohydrate intake. It ensures a constant supply of glucose to the brain, which relies heavily on glucose as its primary fuel source. Gluconeogenesis also prevents the breakdown of muscle protein for energy by providing an alternative source of glucose from amino acids. Furthermore, gluconeogenesis plays a crucial role in the regulation of energy homeostasis. It allows the body to adapt to different metabolic states, such as fasting or exercise, by switching from glucose utilization to fatty acid oxidation. This metabolic flexibility is vital for survival and ensures that the body can efficiently utilize available energy sources.
CONCLUSION OF MECHANISM OF GLUCONEOGENESIS:
Gluconeogenesis is a complex metabolic pathway that allows the body to produce glucose from non-carbohydrate sources. It ensures a steady supply of glucose to meet the energy demands of glucose-dependent tissues, such as the brain. Gluconeogenesis is tightly regulated by hormonal signals and plays a crucial role in maintaining energy homeostasis. Understanding the intricacies of gluconeogenesis provides valuable insights into the body’s backup plan for energy production.
REFERENCES:
Petersen KF, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133-2223. https://pubmed.ncbi.nlm.nih.gov/30067154/
Herzig S, Shaw RJ. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121-135. https://www.sciencedirect.com/science/article/pii/S1097276521006821
Chakraborty M, et al. Gluconeogenesis: An overview of metabolic pathways and their regulation. Diabetes Metab Syndr. 2019;13(1):364-369. https://www.ncbi.nlm.nih.gov/books/NBK544346/
Burgess SC, et al. Mechanisms regulating skeletal muscle glucose metabolism in obesity and type 2 diabetes. Ann Rev Nutr. 2016;36:337-358. https://www.mdpi.com/2072-6643/11/10/2432

