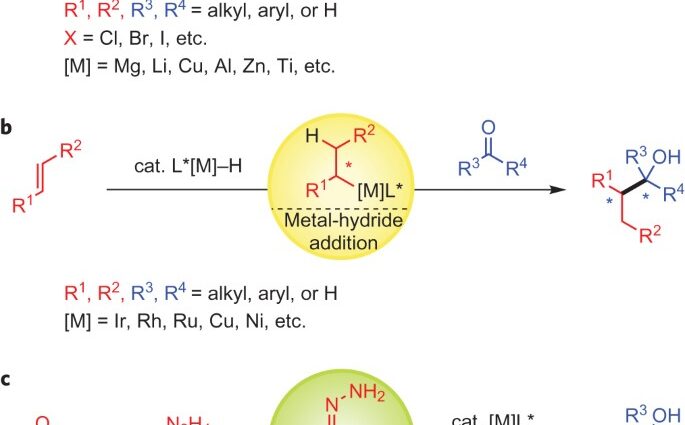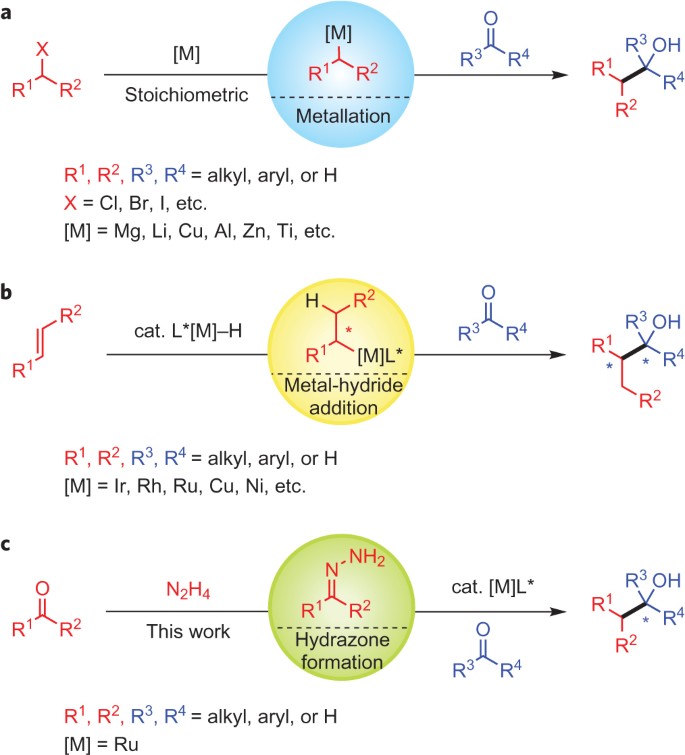
ABSTRACT:
In this article, we will discuss about the synthesis and reactions of carbonyl compounds. Carbonyl compounds are organic compounds with general formula R-C=O-R. There are two basic classes of carbonyl compounds: Aldehydes and ketones. We will also discuss the mechanisms of these reactions. These reactions provide crucial pharmaceutical, industrial and organic products. We will provide references to understand the concept deeply.
INTRODUCTION OF SYNTHESIS AND REACTIONS OF CARBONYL COMPOUNDS:
Carbonyl compounds are a diverse class of organic compounds that contain a carbon-oxygen double bond, known as a carbonyl group (C=O). These compounds play a crucial role in various chemical reactions and found in a wide range of natural and synthetic compounds, including aldehydes, ketones, carboxylic acids, esters, and amides. Understanding the preparations and reactions of carbonyl compounds is essential for organic chemists, as it enables the synthesis of complex molecules and the development of new pharmaceuticals, agrochemicals, and materials.
SYNTHESIS AND REACTIONS OF CARBONYL COMPOUNDS:
1. SYNTHESIS OF CARBONYL COMPOUNDS:
a) OXIDATION OF ALCOHOLS:
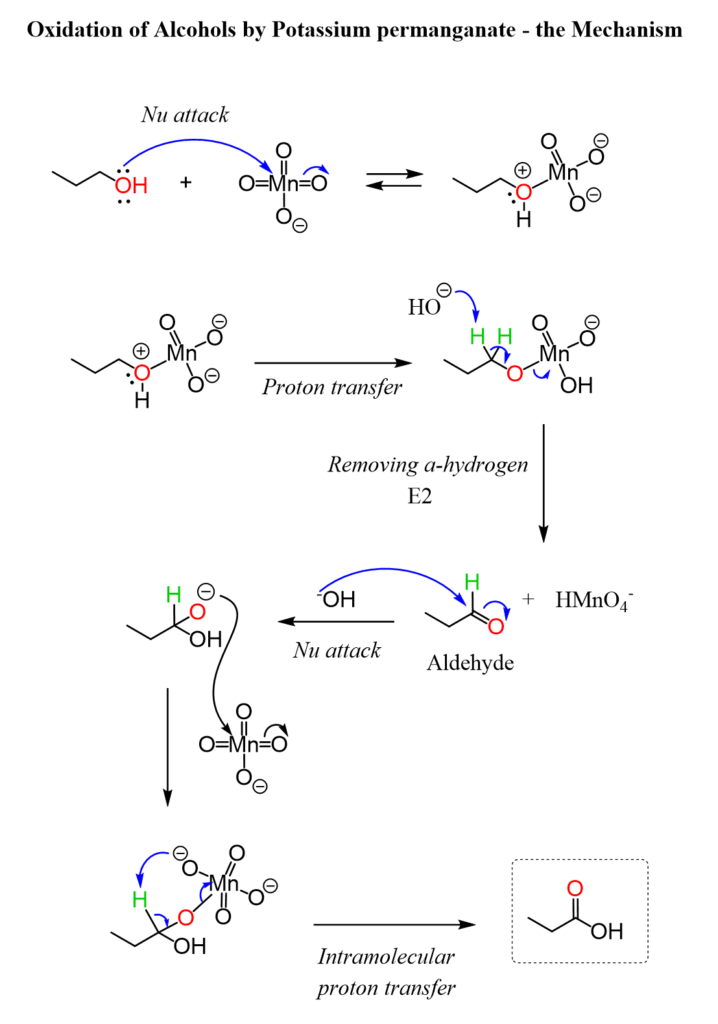
One of the most common methods for preparing carbonyl compounds is the oxidation of primary and secondary alcohols. Primary alcohols oxidized to aldehydes using mild oxidizing agents such as pyridinium chlorochromate (PCC) or the Swern oxidation. Further oxidation of aldehydes leads to carboxylic acids. Secondary alcohols can oxidize to ketones using stronger oxidizing agents like chromic acid (CrO3) or potassium permanganate (KMnO4).
b) OZONOLYSIS OF ALKENES:
Ozonolysis is a powerful method for the preparation of carbonyl compounds from alkenes. The reaction involves the cleavage of the carbon-carbon double bond by ozone (O3), followed by oxidative workup. This process generates aldehydes or ketones, depending on the substitution pattern of the starting alkene.

c) FRIEDEL-CRAFTS ACYLATION:
The Friedel-Crafts acylation reaction allows the introduction of a carbonyl group into an aromatic ring. It involves the reaction of an aromatic compound with an acyl chloride or acid anhydride in the presence of a Lewis acid catalyst, such as aluminum chloride (AlCl3). This reaction is widely used in the synthesis of pharmaceuticals, dyes, and fragrances.

2. REACTIONS OF CARBONYL COMPOUNDS:
a) NUCLEOPHILIC ADDITION:
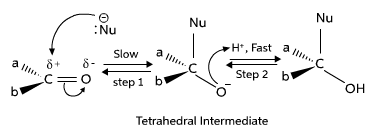
The carbonyl group is highly reactive towards nucleophilic attack due to the polarity of the C=O bond. Nucleophiles, such as water, alcohols, amines, and organometallic reagents, can add to the carbonyl carbon, resulting in the formation of alcohols, hemiacetals, acetals, imines, and enamines, respectively. These reactions are fundamental in the synthesis of various functional groups and complex organic molecules.
b) REDUCTION OF CARBONYL COMPOUNDS:
Carbonyl compounds can be reduced to alcohols using reducing agents like sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4). Aldehydes are typically more reactive towards reduction than ketones. Reduction reactions are widely employed in the synthesis of pharmaceuticals, as they allow the conversion of carbonyl-containing drugs into their corresponding alcohol derivatives, which often exhibit improved pharmacological properties.
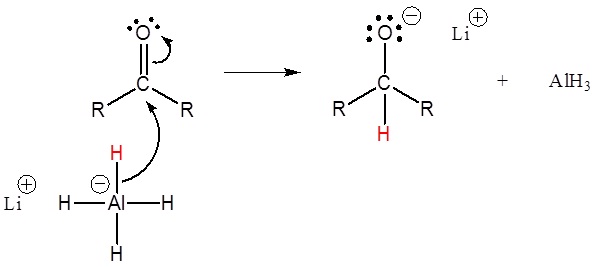
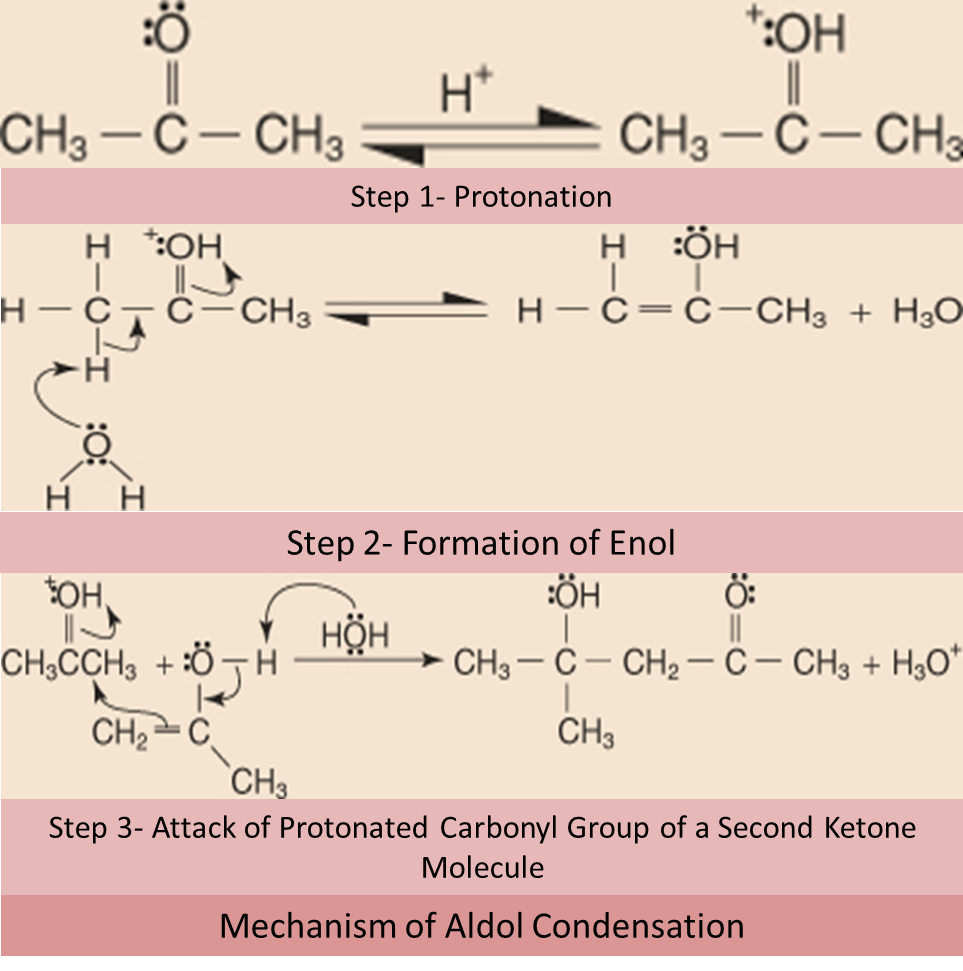
c) CONDENSATION REACTION OF CARBONYL COMPOUNDS:
Carbonyl compounds can undergo condensation reactions, leading to the formation of carbon-carbon or carbon-nitrogen bonds. For example, aldehydes and ketones can react with primary amines to form imines, while aldehydes and ketones with α-hydrogens can undergo aldol condensation to form β-hydroxy carbonyl compounds. These reactions are crucial in the synthesis of natural products, polymers, and pharmaceuticals.
CONCLUSION:
The preparations and reactions of carbonyl compounds are essential tools in organic synthesis, enabling the construction of complex molecules with diverse functionalities. By understanding the mechanisms and applications of these reactions, chemists can design efficient synthetic routes and develop new compounds with desired properties. The versatility of carbonyl compounds makes them indispensable in various fields, including pharmaceuticals, materials science, and agrochemicals.
REFERENCES:
Clayden, J., Greeves, N., Warren, S., & Wothers, P. (2012). Organic Chemistry. Oxford University Press. https://www.chemcome.com/wp-content/uploads/2020/11/Organic-Chemistry-by-Jonathan-Clayden-Nick-Greeves-Stuart-Warren-z-lib.org_.pdf
March, J. (2013). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. John Wiley & Sons. https://rushim.ru/books/mechanizms/march6ed.pdf
Smith, M. B., & March, J. (2007). March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. John Wiley & Sons. https://rushim.ru/books/mechanizms/march6ed.pdf