ABSTRACT:
In this article, we will discuss about the synthesis and reactions of alcohols. Alcohols are organic compounds with general formula R-OH. These reactions provide very crucial organic, pharmaceutical and industrial products. We will also discuss about the mechanisms of these reactions. we will also provide references ton understand the concept deeply.
INTRODUCTION OF SYNTHESIS AND REACTIONS OF ALCOHOLS:
Alcohols are a versatile class of organic compounds that play a crucial role in various industries, including pharmaceuticals, cosmetics, and fuel production. Understanding the preparations and reactions of alcohols is essential for chemists to harness their potential in synthesis and functionalization. This article aims to provide an overview of the different methods used to prepare alcohols and explore their reactions.
SYNTHESIS AND REACTIONS OF ALCOHOLS:
1. SYNTHESIS OF ALCOHOLS:
a) HYDRATION OF ALKENES:
One of the most common methods to prepare alcohols is through the hydration of alkenes. This reaction involves the addition of water to an alkene in the presence of an acid catalyst. The mechanism for this reaction proceeds through a carbocation intermediate, followed by nucleophilic attack of water. For example, the hydration of propene yields propanol: Propene + H2O → Propanol

b) REDUCTION OF CARBONYL COMPOUNDS:
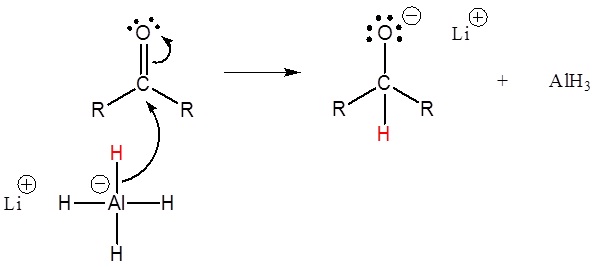
Another important method for alcohol synthesis is the reduction of carbonyl compounds, such as aldehydes and ketones. This reaction can be achieved using reducing agents like sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4). The mechanism involves the transfer of a hydride ion to the carbonyl carbon, followed by protonation. For instance, the reduction of acetone yields isopropanol: Acetone + NaBH4 → Isopropanol
c) GRIGNARD REACTION:
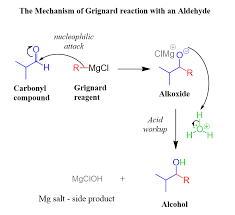
The Grignard reaction is a powerful tool for the preparation of alcohols. It involves the reaction of an alkyl or aryl halide with a Grignard reagent, typically an organo-magnesium compound. The resulting alkoxide intermediate is then protonated to yield the alcohol. The mechanism for this reaction proceeds through the formation of a carbon-carbon bond between the Grignard reagent and the carbonyl carbon, followed by protonation. For example, the reaction of methyl-magnesium bromide with formaldehyde yields methanol: CH2O + CH3MgBr → CH3OH
2. REACTIONS OF ALCOHOLS:
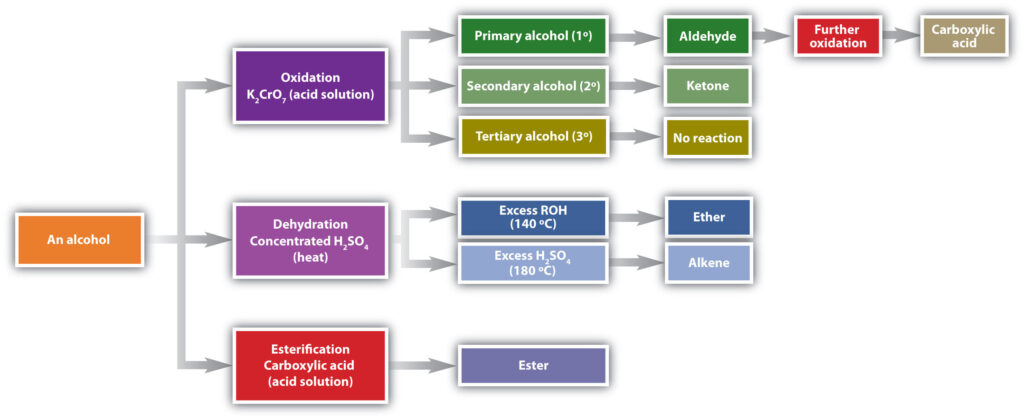
a) OXIDATION OF ALCOHOLS:
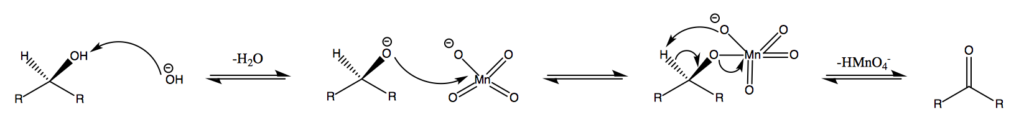
Alcohols can undergo oxidation reactions to form aldehydes, ketones, or carboxylic acids. The choice of oxidizing agent determines the product obtained. For example, primary alcohols can be oxidized to aldehydes using mild oxidizing agents like pyridinium chlorochromate (PCC), while stronger oxidizing agents like potassium permanganate (KMnO4) can further oxidize aldehydes to carboxylic acids.
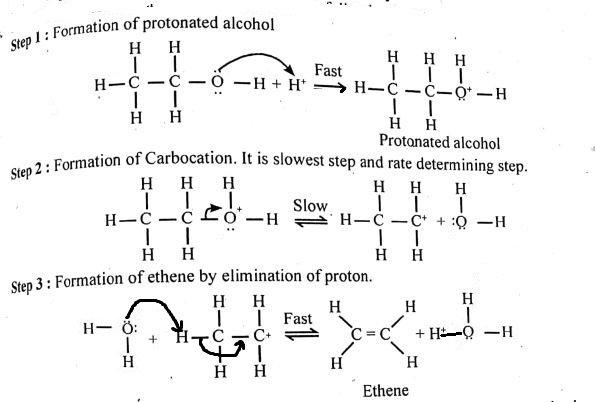
b) DEHYDRATIONS OF ALCOHOLS:
Alcohols can undergo dehydration reactions to form alkenes. This reaction is typically carried out in the presence of an acid catalyst, such as sulfuric acid (H2SO4). The mechanism involves the removal of a water molecule from the alcohol, resulting in the formation of a double bond. For instance, the dehydration of ethanol yields ethene: Ethanol → Ethene + H2O
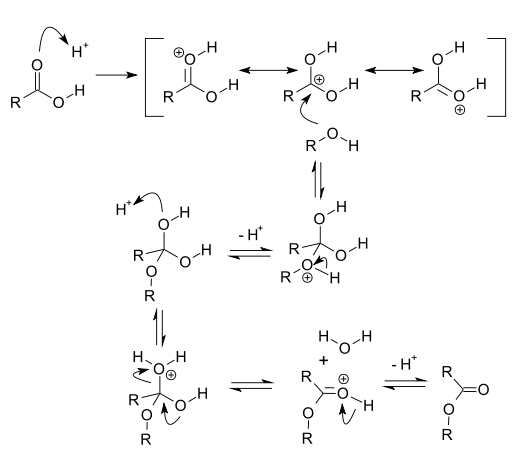
c) ESTERIFICATION OF ALCOHOLS:
Alcohols can react with carboxylic acids to form esters. This reaction is catalyzed by an acid, typically sulfuric acid or hydrochloric acid. The mechanism involves the protonation of the carboxylic acid, followed by nucleophilic attack of the alcohol. For example, the reaction of ethanol with acetic acid yields ethyl acetate: Ethanol + Acetic acid → Ethyl acetate + H2O
APPLICATIONS OF ALCOHOLS:
1. PHARMACEUTICALS:
Alcohols widely used in the pharmaceutical industry as solvents, reagents, and intermediates in the synthesis of various drugs. They also serve as vehicles for drug delivery and as active ingredients in topical medications.
2. COSMETICS:
Alcohols commonly used in cosmetics and personal care products as solvents, preservatives, and fragrance ingredients. They help dissolve other ingredients, improve product stability, and provide a cooling or refreshing sensation.
3. FUEL PRODUCTION:
Alcohols, such as ethanol and methanol, used as alternative fuels or fuel additives due to their renewable nature and lower emissions compared to fossil fuels. Ethanol is commonly blend with gasoline, while methanol used as a fuel in certain vehicles and as a feedstock for biodiesel production.
CONCLUSION:
The preparations and reactions of alcohols offer a wide range of possibilities for organic synthesis and functionalization. Understanding the mechanisms behind these reactions allows chemists to design efficient synthetic routes and explore the diverse applications of alcohols in various industries. By harnessing the potential of alcohols, researchers can contribute to the development of new drugs, cosmetics, and sustainable fuel sources.
REFERENCES:
March, J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. Wiley, 2007. https://rushim.ru/books/mechanizms/march6ed.pdf
Smith, M. B., & March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. Wiley, 2013. https://rushim.ru/books/mechanizms/march6ed.pdf
Clayden, J., Greeves, N., & Warren, S. Organic Chemistry. Oxford University Press, 2012. https://iroch.ir/wp-content/uploads/2020/12/Clayden-Organic-Chemistry-2nd-edition-c2012-txtbk.pdf
Li, J. J. Name Reactions: A Collection of Detailed Reaction Mechanisms. Springer, 2006.


