ABSTRACT:
In this article, we will thoroughly explore the crystal field theory and its application in studying the transition metal complexes. Crystal field theory provides a framework for understanding the behavior and properties of these complexes through the analysis of the interaction between the transition metal ion and its surrounding ligands. By delving deeper into the concepts of ligand field effects and crystal field splitting, we can gain valuable insights into the electronic structure and spectroscopic properties of transition metal complexes. Moreover, this theory also has significant implications in various fields such as bioinorganic chemistry, materials science, and coordination chemistry. With a comprehensive understanding of the crystal field theory, we can uncover new possibilities and advancements in the study of transition metal complexes.
INTRODUCTION OF CRYSTAL FIELD THEORY:
In the field of chemistry, the study of transition metal complexes plays crucial role in understanding various chemical phenomena. Transition metals exhibit unique properties due to the presence of partially filled d-orbitals. These properties allow them to form coordination complexes with ligands. Crystal Field Theory (CFT), a widely accepted model that explains the electronic structure and properties of these complexes. This article aims to provide an overview of CFT, its key principles, and its significance. This will help in understanding the behavior of transition metal complexes. https://www.sciencedirect.com/science/article/pii/S2590147822000535
PRINCIPLES OF CRYSTAL FIELD THEORY:
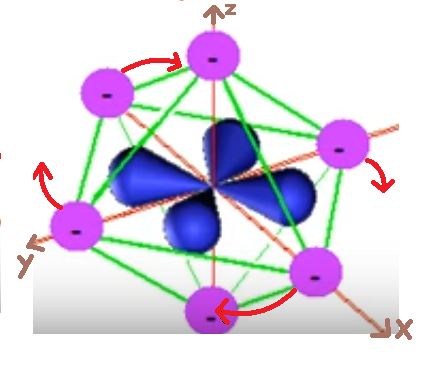
Crystal Field Theory based on the assumption that the ligands surrounding a transition metal ion can treated as point charges. These ligands generate an electrostatic field that affects the energy levels of the d-orbitals of the central metal ion. The interaction between the metal ion and the ligands can divided into two components: the electrostatic repulsion and the ligand field stabilization energy (LFSE).
1. ELECTROSTATIC REPULSION:
The negatively charged ligands repel the negatively charged electrons in the d-orbitals of the metal ion. This repulsion leads to a splitting of the d-orbitals into two sets of energy levels: the lower energy set (eg) and the higher energy set (t2g). The energy difference between these sets is known as the crystal field splitting energy (Δ).

2. LIGAND FIELD STABILIZATION ENERGY:
The presence of ligands also leads to a stabilization of the d-orbitals. The electrons in the lower energy set (eg) experience less repulsion from the ligands and are therefore more stable. This stabilization energy is known as the Ligand Field Stabilization Energy (LFSE). The LFSE is responsible for the observed colors, magnetic properties, and reactivity of transition metal complexes.

SIGNIFICANCE OF CRYSTAL FIELD THEORY:
Crystal Field Theory provides a framework for understanding various properties of transition metal complexes:
1. COLOR:
The absorption of light by transition metal complexes is directly related to the crystal field splitting energy (Δ). The energy difference between the eg and t2g orbitals corresponds to specific wavelengths of light, resulting in the observed colors. For example, a complex with a high crystal field splitting energy (large Δ) absorbs light in the blue region of the spectrum, while a complex with a low crystal field splitting energy (small Δ) absorbs light in the red region.

2. MAGNETIC PROPERTIES OF CRYSTAL FIELD THEORY:
The number of unpaired electrons in the d-orbitals of a transition metal complex determines its magnetic properties. In CFT, complexes with unpaired electrons in the t2g orbitals are classified as high spin, while those with unpaired electrons in the eg orbitals are classified as low spin. This distinction has implications for the magnetic behavior of the complexes.

3. REACTIVITY:
The crystal field splitting energy (Δ) also influences the reactivity of transition metal complexes. The energy required to promote an electron from the lower energy t2g orbitals to the higher energy eg orbitals affects the ease with which the complex can undergo redox reactions or bond with other ligands.
CONCLUSION:
Crystal Field Theory is a fundamental model that provides insights into the electronic structure and properties of transition metal complexes. By considering the electrostatic repulsion and ligand field stabilization energy, CFT explains the observed colors, magnetic properties, and reactivity of these complexes. Understanding CFT is crucial for designing catalysts, understanding biological processes, and developing new materials with tailored properties.
REFERENCES:
Cotton, F. A., & Wilkinson, G. (1988). Advanced Inorganic Chemistry. Wiley. https://chemistlibrary.files.wordpress.com/2015/05/cotton-wilkinson-advanced-inorganic-chemistry.pdf
Huheey, J. E., Keiter, E. A., & Keiter, R. L. (1993). Inorganic Chemistry: Principles of Structure and Reactivity. HarperCollins College Publishers.
Miessler, G. L., & Tarr, D. A. (2013). Inorganic Chemistry. Pearson Education.

