
ABSTRACT:
In this article, we will discuss about the synthesis and reactions of alkyl halides. Alkyl halides also called haloalkanes due to presence of halogen atoms. These are very reactive and highly electronegative organic compounds. We will discuss about the mechanisms of different reactions of alkyl halides. We will also provide references to understand our concept deeply.
INTRODUCTION OF SYNTHESIS AND REACTIONS OF ALKYL HALIDES:
Alkyl halides, also known as haloalkanes, are organic compounds that contain a halogen atom (fluorine, chlorine, bromine, or iodine) bonded to a carbon atom. These compounds play a crucial role in organic synthesis and widely used in various industries. Understanding the preparations, reactions, and mechanisms of alkyl halides is essential for chemists to design and optimize synthetic routes and develop new applications.
SYNTHESIS AND REACTIONS OF ALKYL HALIDES:
1. SYNTHESIS OF ALKYL HALIDES:
There are several methods to prepare alkyl halides, depending on the starting materials and desired halogen substitution. Some common methods include:
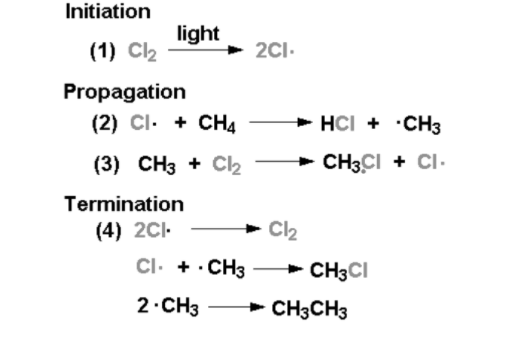
a) HALOGENATION OF ALKANES:
Alkanes can halogenated by reacting them with halogens (Cl2, Br2, or I2) or hydrogen halides (HCl, HBr, or HI) in the presence of a catalyst such as iron or UV light. This method is suitable for the preparation of primary and secondary alkyl halides.
b) NUCLEOPHILIC SUBSTITUTION OF ALCOHOLS:
Alcohols can convert into alkyl halides through nucleophilic substitution reactions. The alcohol reacts with a hydrogen halide (HX) or a phosphorus halide (PX3 or PCl5) to form the corresponding alkyl halide. This method commonly used for the preparation of tertiary alkyl halides.

c) ADDITION OF HALOGENS TO ALKENES:
Alkenes can undergo halogenation reactions, where the double bond is broken, and halogen atoms are added to the carbon atoms. This reaction is typically carried out using halogens (Br2 or Cl2) or hydrogen halides (HBr or HCl) as the halogen source. The addition of halogens to alkenes results in the formation of vicinal dihalides.

2. REACTIONS OF ALKYL HALIDES:
Alkyl halides participate in a wide range of reactions due to the presence of the electrophilic carbon-halogen bond. Some important reactions include:
a) NUCLEOPHILIC SUBSTITUTION REACTIONS:
Alkyl halides can undergo nucleophilic substitution reactions, where a nucleophile replaces the halogen atom.
1. SN1 REACTION:
In this reaction, the nucleophile attacks the carbocation formed from the alkyl halide. The reaction typically proceeds in two steps involving the formation of a reactive carbocation intermediate. SN1 reactions are favored in the presence of a weak nucleophile and a polar solvent.

2. SN2 REACTION:
The nucleophile directly displaces the leaving group in a single concerted step. These reactions proceed through a backside attack, resulting in an inversion of stereochemistry. SN2 reactions are favored with primary alkyl halides and strong nucleophiles.

b) ELIMINATION REACTIONS:
Alkyl halides can undergo elimination reactions, where a halogen atom is eliminated along with a proton, resulting in the formation of a double bond.
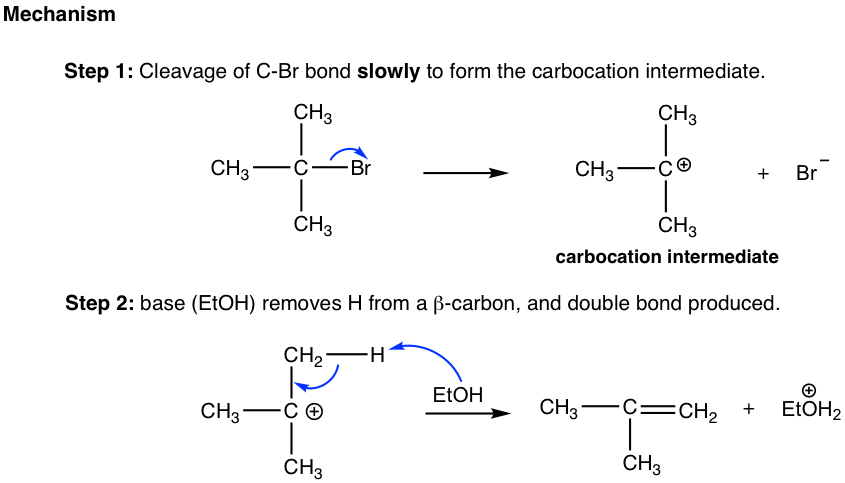
1. E1 REACTION:
In this reaction, the elimination of a leaving group occurs simultaneously with the formation of a carbocation intermediate. E1 reactions are favored when the alkyl halide is tertiary or secondary and in the presence of a weak base.
2. E2 REACTION:
The elimination of a leaving group and the formation of a double bond occur in a concerted and single-step process. Unlike the E1 mechanism, E2 reactions occur with primary and secondary alkyl halides and in the presence of strong bases.

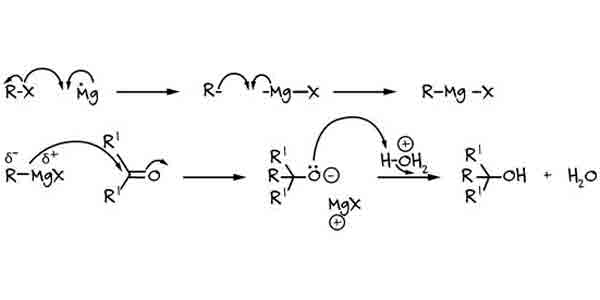
c) GRIGNARD REAGENT REACTIONS:
Alkyl halides can react with magnesium metal to form organomagnesium compounds known as Grignard reagents. These reagents are highly reactive and can be used for the synthesis of alcohols, aldehydes, ketones, and carboxylic acids.
CONCLUSION:
Alkyl halides are versatile compounds that can be prepared through various methods, including halogenation of alkanes, nucleophilic substitution, and addition of halogens to alkenes. These compounds participate in a wide range of reactions, such as nucleophilic substitution, elimination, and Grignard reactions. Understanding the mechanisms of these reactions is crucial for designing efficient synthetic routes and developing new applications for alkyl halides. The references provided in this article serve as valuable resources for further exploration of this topic.
REFERENCES:
McMurry, J. Organic Chemistry, 9th Edition; Brooks Cole: Boston, 2015. https://gtu.ge/Agro-Lib/McMurry%20J.E.%20-%20Fundamentals%20of%20Organic%20Chemistry,%207th%20ed.%20-%202010.pdf
Clayden, J.; Greeves, N.; Warren, S.; Wothers, P. Organic Chemistry, 2nd Edition; Oxford University Press: Oxford, 2012. https://iroch.ir/wp-content/uploads/2020/12/Clayden-Organic-Chemistry-2nd-edition-c2012-txtbk.pdf
March, J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6th Edition; Wiley: Hoboken, 2007.



Hey there! Your blog is an incredible resource for anyone interested in bingads . Your infographics are incredibly informative and have guided us in our own efforts to grow in the field. We especially loved your recent posts about automotive . Keep up the fantastic work and we look forward to reading more from you soon!
Thanks again this was a great read
Legendary Business Ventures
Marketer
http://www.clickedprofits.co.uk