ABSTRACT:
In this article, we will discuss about the synthesis and reactions of alkenes. Alkenes are unsaturated hydrocarbons with general formula CH2n. We will discuss the mechanism of these synthesis and reactions. These reactions are very crucial for the synthesis of various organic compounds, including pharmaceuticals, polymers and fuels. We will provide references to understand our concepts.
INTRODUCTION:
Alkenes are unsaturated hydrocarbons that play a significant role in organic chemistry due to their versatile reactivity. These compounds characterized by a carbon-carbon double bond, which imparts unique chemical properties. This article aims to provide an overview of the preparations and reactions of alkenes, highlighting the underlying mechanisms and their applications in various fields.
SYNTHESIS AND REACTIONS OF ALKENES:
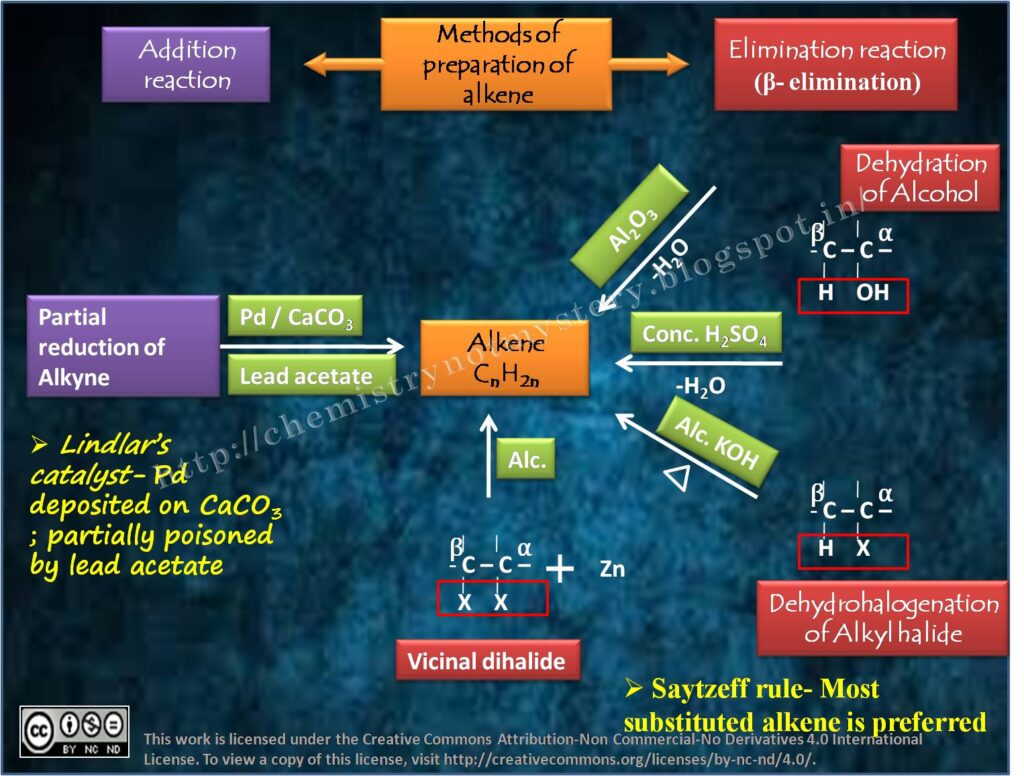
1. SYNTHESIS OF ALKENES:
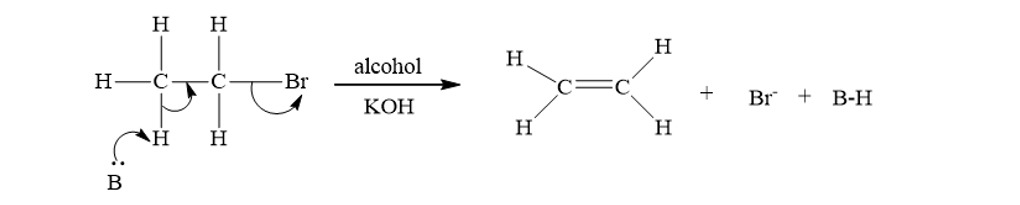
a) DEHYDROHALOGENATION OF ALKYL HALIDES:
Alkenes can prepare by the elimination of a hydrogen halide (H-X) from an alkyl halide. This reaction commonly carried out using a strong base, such as potassium hydroxide (KOH) or sodium hydroxide (NaOH), in an alcohol solvent. The mechanism involves the formation of an alkoxide ion, which subsequently abstracts a proton, leading to the formation of an alkene.
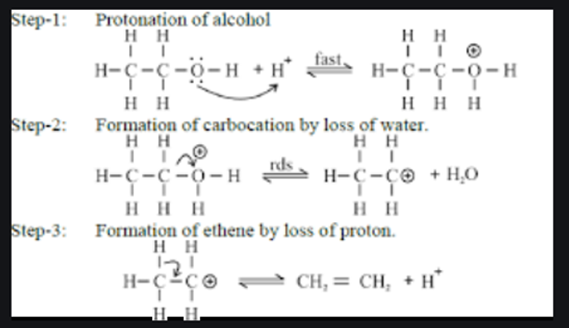
b) DEHYDRATION OF ALCOHOLS:
Alkenes can synthesize by the dehydration of alcohols, where a molecule of water eliminated from an alcohol molecule. This reaction typically catalyzed by an acid, such as sulfuric acid (H2SO4) or phosphoric acid (H3PO4). The mechanism involves the formation of a carbocation intermediate, followed by the loss of a proton to yield the alkene.
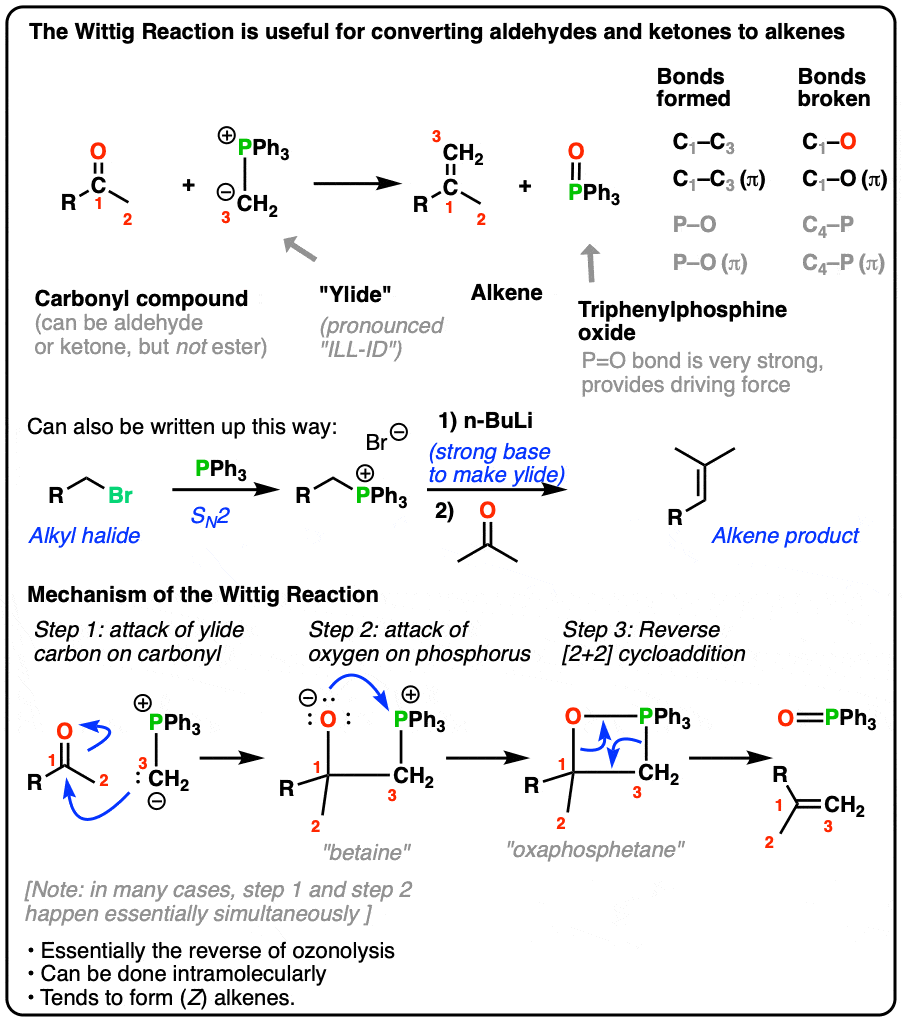
c) WITTIG REACTION:
The Wittig reaction allows the synthesis of alkenes from aldehydes or ketones using a phosphorus ylide as a reagent. The ylide reacts with the carbonyl compound to form a betaine intermediate, which then undergoes a concerted [2+2] cycloaddition reaction, resulting in the formation of an alkene.
2. REACTIONS OF ALKENES:
a) ADDITION REACTIONS:
Alkenes readily undergo addition reactions due to the presence of a reactive π bond. Some important addition reactions include:
1. HYDROGENATION OF ALKENES:
Alkenes can be selectively hydrogenated using a catalyst, such as palladium on carbon (Pd/C), to yield the corresponding alkane.

2. HALOGENATION OF ALKENES:
Alkenes react with halogens (Cl2, Br2) to form dihalides. The reaction proceeds through a cyclic bromonium or chloronium ion intermediate.

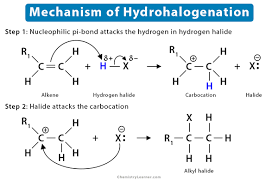
3. HYDROHALOGENATION OF ALKENES:
Alkenes react with hydrogen halides (HCl, HBr) to yield alkyl halides. The reaction follows Markovnikov’s rule, where the hydrogen atom adds to the carbon atom with fewer hydrogen substituents.
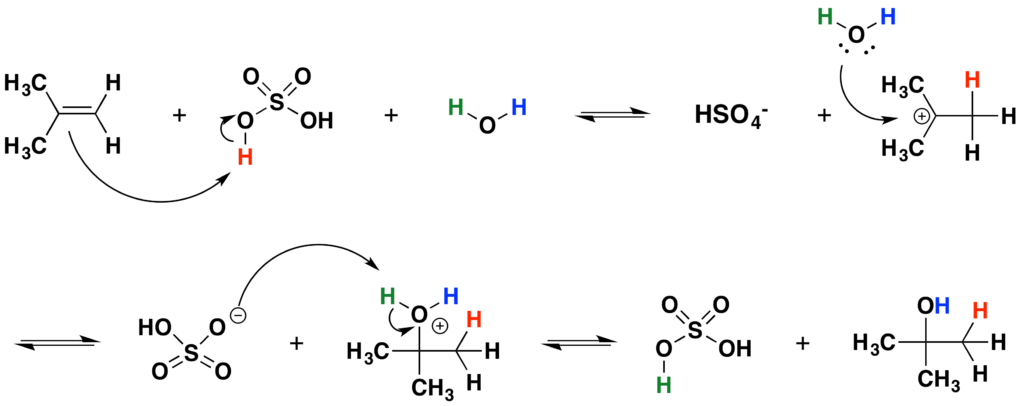
4. HYDRATION OF ALKENES:
Alkenes can be hydrated in the presence of acid catalysts to form alcohols. This reaction is commonly known as the addition of water across the double bond.
b) OXIDATION REACTIONS OF ALKENES:
Alkenes can undergo oxidation reactions to form various functional groups. Notable examples include:
1. EPOXIDATION:
Alkenes react with peroxides, such as m-chloroperbenzoic acid (mCPBA), to form epoxides. This reaction involves the formation of an intermediate peroxycarboxylic acid, which then reacts with the alkene to yield the cyclic ether.

2. OZONOLYSIS:
Alkenes can be cleaved into two carbonyl compounds by treatment with ozone (O3) followed by a reducing agent, such as zinc (Zn) or dimethyl sulfide (CH3)2S.

CONCLUSION:
Understanding the preparations and reactions of alkenes is crucial for organic chemists and researchers. This article provided an overview of the major methods for preparing alkenes, including dehydrohalogenation, dehydration, and the Wittig reaction. Additionally, it discussed important addition and oxidation reactions of alkenes, highlighting their mechanisms and applications in various synthetic processes. By mastering these reactions, chemists can harness the reactivity of alkenes for the synthesis of complex organic molecules.
REFERENCES:
Clayden, J., Greeves, N., & Warren, S. (2012). Organic Chemistry. Oxford University Press. https://global.oup.com/academic/product/organic-chemistry-9780199270293?cc=pk&lang=en&
Smith, M. B., & March, J. (2007). March’s Advanced Organic Chemistry. Wiley-Interscience. https://rushim.ru/books/mechanizms/march6ed.pdf
Carey, F. A., & Sundberg, R. J. (2007). Advanced Organic Chemistry: Part A: Structure and Mechanisms. Springer Science & Business Media. https://chemistlibrary.files.wordpress.com/2015/07/advanced-organic-chemistry-4ed-2000-part-a-structure-and-mechanisms-carey-sundberg.pdf
Klein, D. R. (2011). Organic Chemistry as a Second Language: First Semester Topics. John Wiley & Sons.


