ABSTRACT:
In this article, we will discuss about the phenomenon of resonance and resonating structures. The delocalization of pi electrons within the molecules called resonance. Resonance structures are the canonical forms of the molecules and are combination of more than one structures. This phenomenon play vital role in determining the stability and reactivity of molecules. We will discuss the significance and applications of resonance.
INTRODUCTION OF RESONANCE AND RESONANCE STRUCTURES:
Resonance is a fundamental concept in chemistry that helps explain the stability and reactivity of molecules. It occurs when a molecule can represented by multiple Lewis structures, known as resonating structures, which differ only in the arrangement of electrons. This article aims to provide a comprehensive understanding of resonance and resonating structures, exploring their significance in chemical bonding and reactivity.
RESONANCE IN CHEMICAL BONDING:
In traditional Lewis structures, atoms connected by single, double, or triple bonds. However, some molecules cannot accurately represented by a single Lewis structure due to the delocalization of electrons. Resonance allows us to describe these molecules more accurately by considering all possible arrangements of electrons.

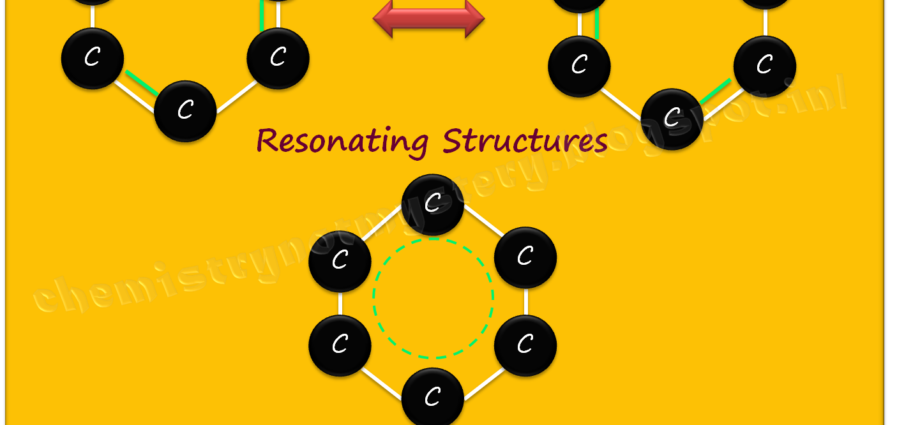
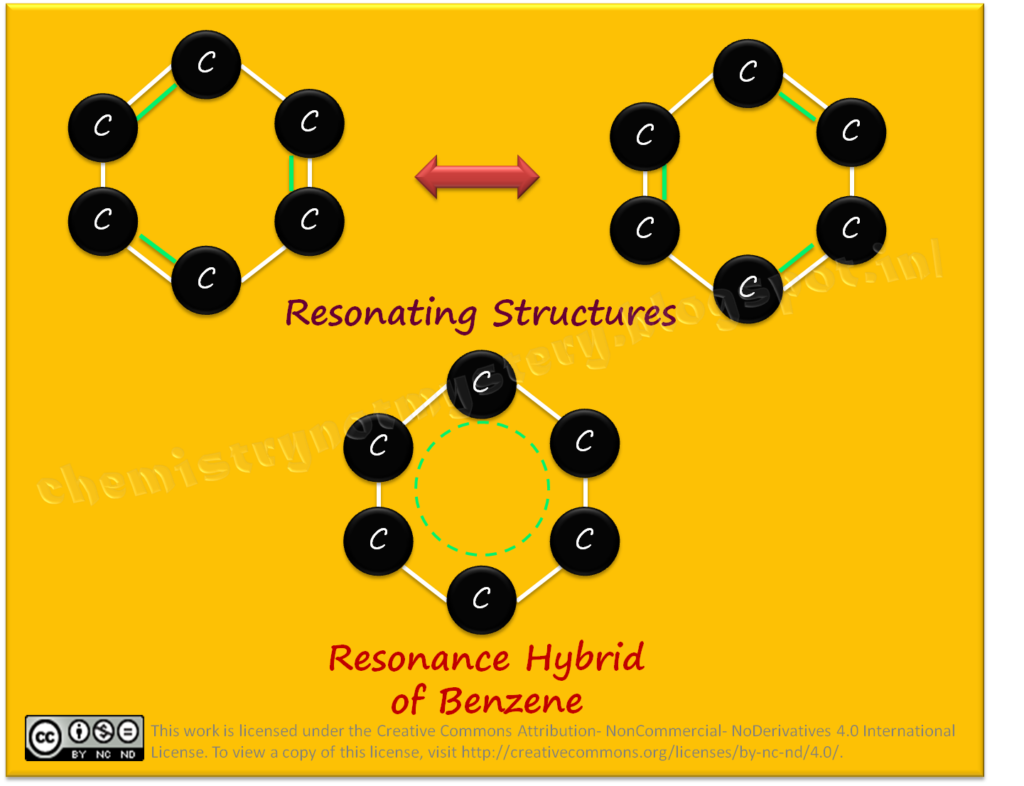
RESONATING STRUCTURES:
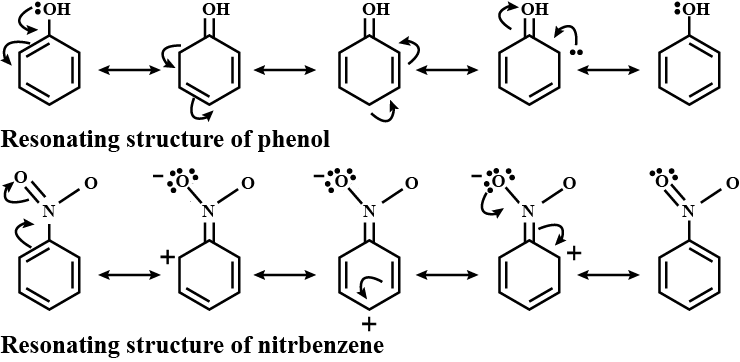
Resonating structures are different representations of a molecule that can interconverted by moving electrons. These structures connected by double-headed arrows to indicate their equivalence. It is important to note that resonating structures do not exist as separate entities but rather represent the true nature of the molecule, which is a hybrid of all the possible structures.
SIGNIFICANCE OF RESONANCE AND RESONATING STRUCTURES:
Resonance plays a crucial role in determining the stability and reactivity of molecules. It helps explain phenomena such as the delocalization of charge, the distribution of electron density, and the stability of certain compounds. By considering all possible resonating structures, we can better understand the behavior of molecules in chemical reactions.
1. DELOCALIZATION OF CHARGE:
Resonance allows for the delocalization of charge within a molecule. This means that electrons are not confined to a specific atom or bond but are spread out over multiple atoms. This delocalization stabilizes the molecule by distributing the charge more evenly, reducing the repulsion between electrons.

2. DISTRIBUTION OF ELECTRON DENSITY:
Resonance also affects the distribution of electron density within a molecule. In resonating structures, electrons are not localized in specific bonds but are shared among multiple bonds. This results in a more uniform distribution of electron density, making the molecule more stable.
3. STABILITY OF COMPOUNDS:
Molecules with resonating structures are generally more stable than those without. This is because the delocalization of electrons and the distribution of electron density contribute to a lower overall energy state. For example, benzene, a molecule with a resonance structure, is exceptionally stable due to the delocalization of its π electrons.

APPLICATIONS OF RESONANCE AND RESONANCE STRUCTURES:
The concept of resonance finds applications in various areas of chemistry. It helps explain the stability of certain compounds, the reactivity of molecules in organic reactions, and the behavior of radicals and ions. Resonance is also crucial in understanding the properties of conjugated systems, such as conjugated dienes and aromatic compounds.
CONCLUSION:
Resonance and resonating structures are essential concepts in chemistry that provide a more accurate representation of molecules. By considering all possible arrangements of electrons, resonance helps explain the stability and reactivity of compounds. Understanding resonance is crucial for predicting the behavior of molecules in chemical reactions and has significant applications in various fields of chemistry.
REFERENCES:
Clayden, J., Greeves, N., Warren, S., & Wothers, P. (2012). Organic Chemistry. Oxford University Press. https://www.chemcome.com/wp-content/uploads/2020/11/Organic-Chemistry-by-Jonathan-Clayden-Nick-Greeves-Stuart-Warren-z-lib.org_.pdf
McMurry, J. (2015). Organic Chemistry. Cengage Learning. https://pubs.acs.org/doi/abs/10.1021/acs.jchemed.8b00137
Vollhardt, K. P. C., & Schore, N. E. (2014). Organic Chemistry: Structure and Function. W. H. Freeman and Company. https://pubs.rsc.org/en/content/articlelanding/2014/nr/c4nr02544j/unauth